RELiZORB is proven in clinical studies to break down fats in enteral formula

RELiZORB normalizes absorption of fatty acids with immediate use1
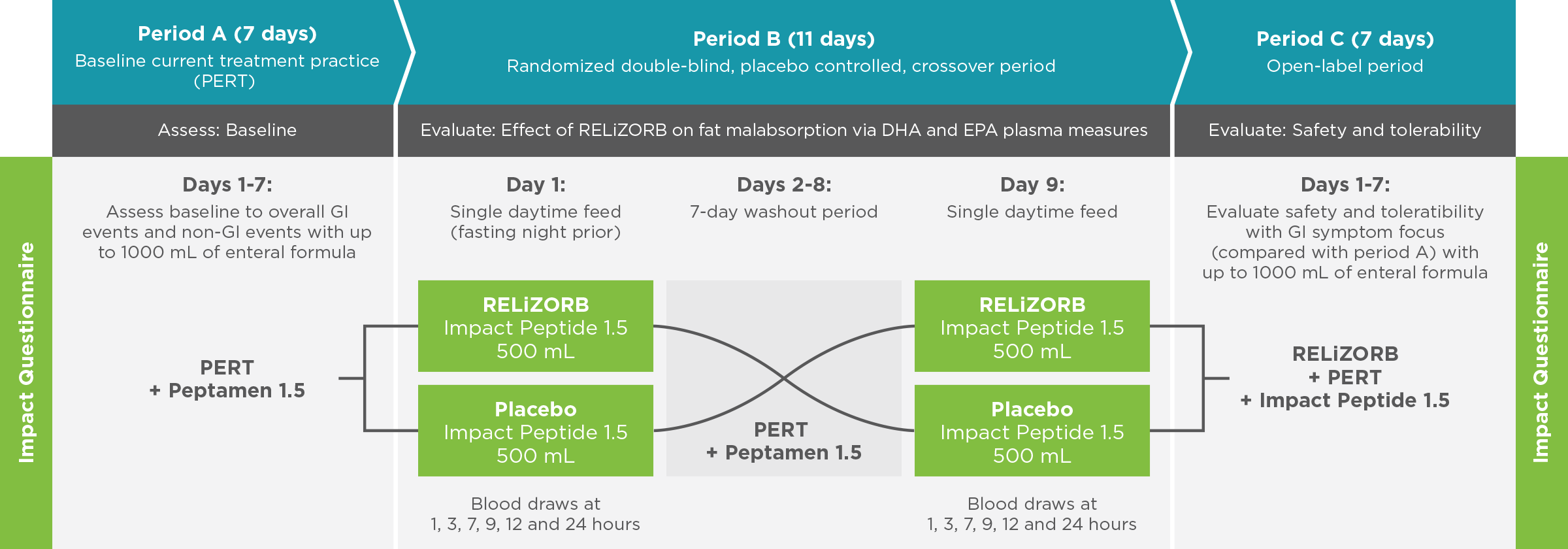
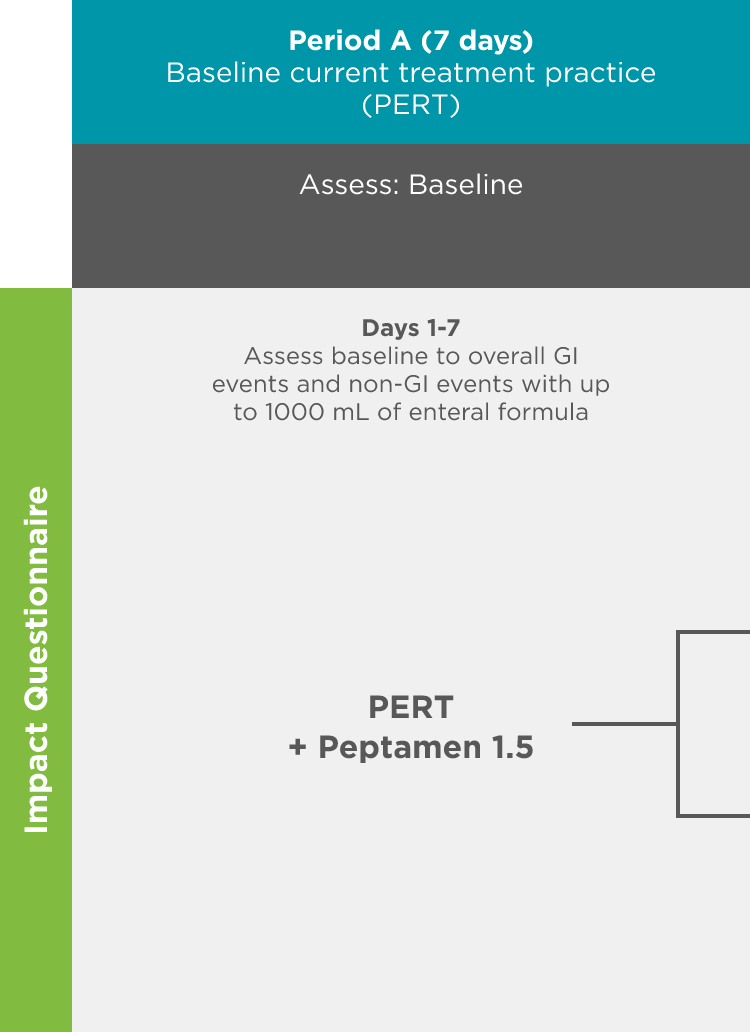
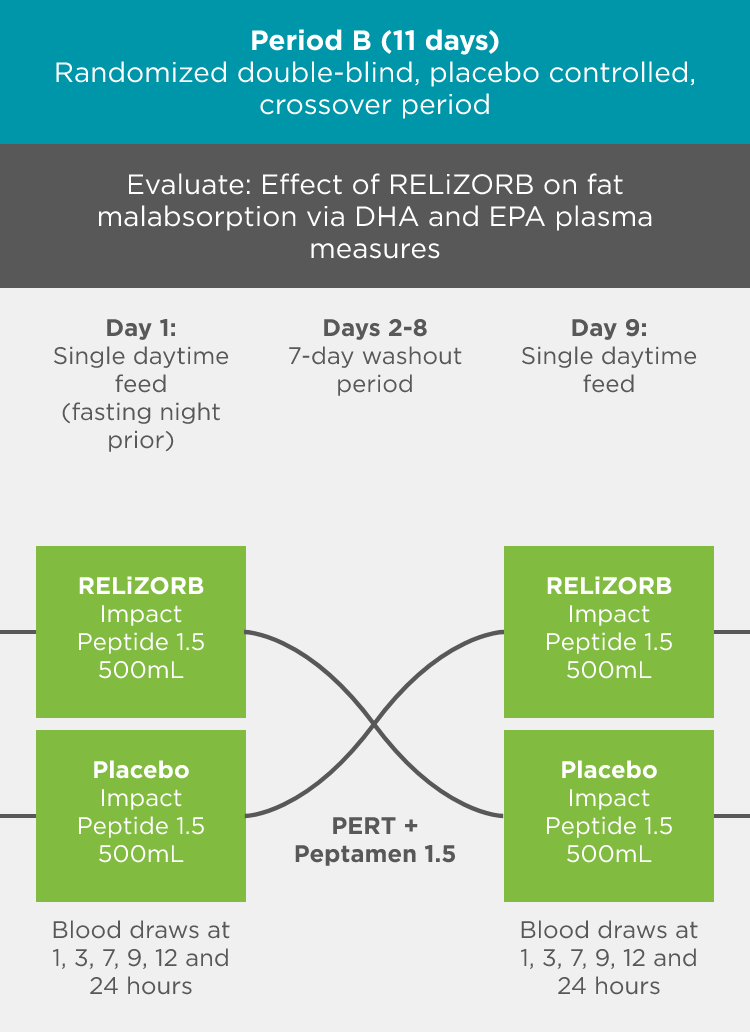
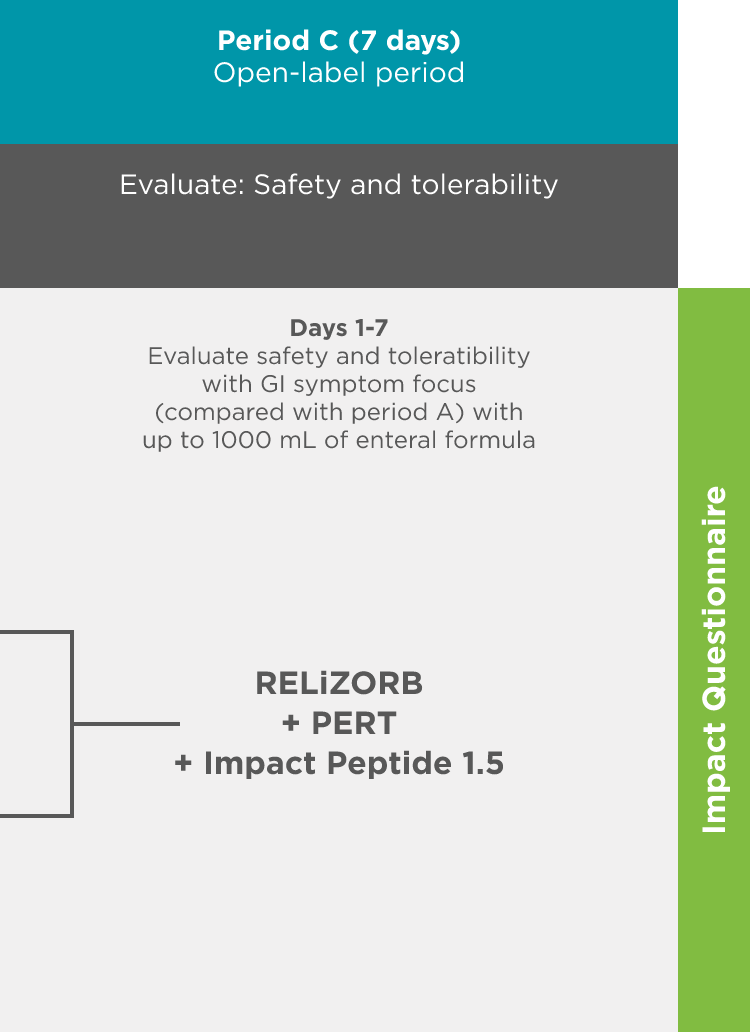
497 Study: A double-blind, placebo-controlled crossover treatment period with a follow-up safety period was used to measure endpoints1
Objective: Evaluate the safety, tolerability, and fat absorption with RELiZORB in patients with exocrine pancreatic insufficiency due to cystic fibrosis.
- The efficacy endpoint was fat absorption as measured by plasma concentrations of DHA and EPA.
- An assessment of safety and tolerability was completed using a symptom diary and impact questionnaire.
Over 24 hours: changes in plasma concentrations of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) with RELiZORB1
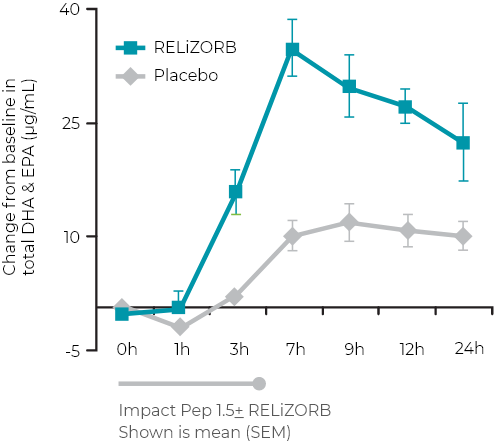
2.8-fold overall increase in total DHA and EPA with RELiZORB versus placebo (AUC0-24h; P<0.001)1
Use of RELiZORB was shown to normalize* plasma concentrations of DHA and EPA1
DHA and EPA were used as measures in the studies as they are strongly correlated with overall fat absorption.2
*Use of RELiZORB was shown to normalize concentrations to levels consistent with a reference range based on healthy subjects as shown in the literature.
Decreased frequency of gastrointestinal (GI) symptoms with RELiZORB1
In the 497 study, overall, the frequency of GI events decreased among 33 pediatric and adult patients with cystic fibrosis1
During Period C of the trial, 42% (n=14) of patients using RELiZORB stopped taking pancreatic enzyme replacement therapy (PERT) capsules, despite protocol instructions to maintain their usual treatment practice.1
RELiZORB vs. PERTs alone: see the GI symptom comparison data1
| OVERALL (n=33) | PERT (Period A) |
PERT + RELiZORB (Period C) |
|---|---|---|
| ABDOMINAL PAIN | 29 (13) | 19 (10) |
| BLOATING | 14 (5) | 7 (3) |
| CONSTIPATION | 8 (6) | 0 (0) |
| DIARRHEA | 7 (7) | 3 (3) |
| GAS | 30 (12) | 38 (10) |
| INDIGESTION / HEARTBURN | 9 (6) | 4 (3) |
| NAUSEA | 9 (6) | 6 (4) |
| STEATORRHEA / FATTY STOOL | 7 (6) | 7 (3) |
| VOMITING | 4 (3) | 5 (3) |
| FLATULENCE | 1 (1) | 7 (1) |
| SMELLY BURPS | 4 (1) | 0 (0) |
| LARGE VOLUME STOOL | 0 (0) | 4 (2) |
| ABDOMINAL GAS PAIN | 0 (0) | 1 (1) |
| TOTAL FREQUENCY | 122 | 101 |
GI events are expressed as: number of events (number of patients reporting events). Overall the frequency of GI events decreased in Period C compared with Period A among 33 pediatric and adult patients with cystic fibrosis. Period A=baseline run-in period. Period C=open-label safety period. Gastrointestinal events are expressed as: number of events (number of patients reporting events).
RELiZORB normalizes absorption of fatty acids with sustained use3
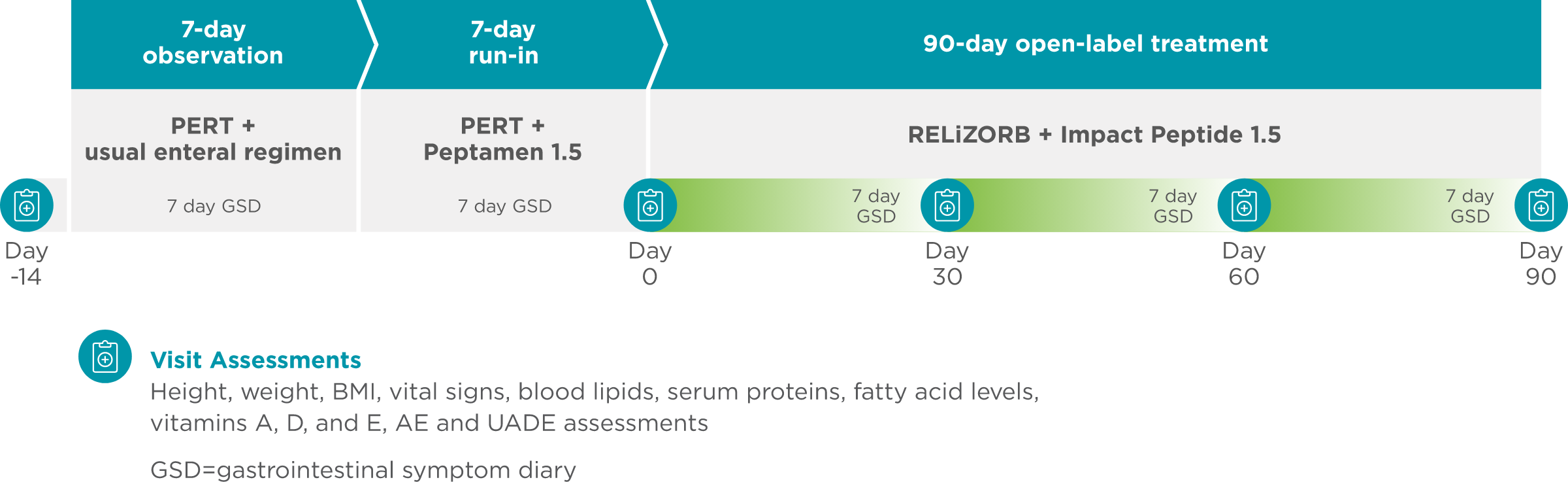
498 ASSURE Study: A multicenter, 90-day, open-label study in which RELiZORB was used with overnight enteral nutrition3
Objective: Evaluate safety, tolerability, and improvement in fatty acid status in red blood cell membranes with RELiZORB in patients with exocrine pancreatic insufficiency due to cystic fibrosis.
- The primary study endpoint was change over time in red blood cell uptake of DHA and EPA relative to total fatty acid composition in erythrocyte membranes (Omega-3 index).
- An assessment of safety tolerability including GI symptoms and adverse events.
- Visit assessments at days 0, 30, 60, and 90 measured height, weight, BMI, vital signs, blood lipids, serum proteins, fatty acid levels, vitamins A, D, and E, adverse events, and unanticipated adverse device effects.
Changes in erythrocyte membrane fatty acid composition (%) for omega-3 index3
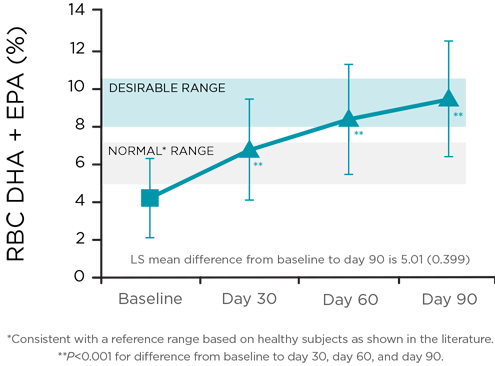
2.1-fold increase in red blood cell uptake of DHA and EPA relative to fatty acid composition in erythrocyte membranes3
Statistically significant increases observed at Day 60 and Day 90 (P<0.001 for each)
In the ASSURE study, longer-term and sustained use of RELiZORB with regular overnight enteral nutrition normalized plasma omega-3 levels consistent with a reference range based on healthy subjects as shown in the literature. Levels were maintained over longer periods, indicating an improvement in fat malabsorption.
Improvement in weight percentiles with RELiZORB3
In the ASSURE study, usage of RELiZORB showed:
- No reported incidences of diarrhea at Day 90
- Overall, the number of participants reporting GI symptoms decreased from Day 30 to Day 90
- RELiZORB is well-tolerated, no participants discontinued RELiZORB due to an adverse event
61% of participants demonstrated improvement in weight percentiles3
Overall, weight and body mass index (BMI) z-scores and percentiles were not significantly different from baseline to 90 days. However, 20/33 (61%) patients had improvement in weight z-scores and percentiles in the intention to treat (ITT) population.
All exploratory efficacy outcomes, including serum levels of fat-soluble vitamins A, D, and E in plasma, as well as serum protein (total protein, prealbumin, albumin, and transferrin) levels, were within normal ranges at study entry and remained so throughout the 90-day study treatment period.
Patients get more from enteral formula
RELiZORB is proven to hydrolyze available fats,* including medium-chain triglycerides (MCTs) and long-chain triglycerides (LCTs).4
- LCTs are complex and contain essential fatty acids5
- Compared to MCTs, LCTs are harder to hydrolyze but provide ~10% more calories5
- RELiZORB is intended to provide continuous fat hydrolysis during enteral feeding
Please see list of compatible formulas.

References
- Freedman S, Orenstein D, Black P, et al. Increased fat absorption from enteral formula through an in-line digestive cartridge in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2017;65(1):97-101.
- Harris WS, Sands SA, Windsor SL, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 2004;110:1645-1649.
- Stevens J, Wyatt C, Brown P, Patel D, Grujic D, Freedman SD. Absorption and Safety with Sustained Use of RELiZORB Evaluation (ASSURE) study in patients with cystic fibrosis receiving enteral feeding. J Pediatr Gastroenterol Nutr. 2018;Oct;67(4):527-532.
- Freedman S. Options for Addressing Exocrine Pancreatic Insufficiency in Patients Receiving Enteral Nutrition Supplementation. Am J Manag Care. 2017 Jul;23(12 Suppl):S220-S228.
- Shah ND, Limketkai BN. The Use of Medium-Chain Triglycerides in Gastrointestinal Disorders. Practical Gastroenterology. 2017 Feb;41(2):20-28.