Step-by-step instructions on setting up and using RELiZORB with your enteral tube feeding set
RELiZORB can be set up for single or tandem use based on the amount of formula volume you need
- For volumes up to 500 mL, connect 1 RELiZORB cartridge to the end of the primed feeding pump tubing set. For volumes greater than 500 mL and up to 1000 mL, connect 2 RELiZORB cartridges in a tandem configuration, then connect to the end of the primed feeding pump tubing set. Up to 2 RELiZORB cartridges can be used in a day (24-hour period).
- Connect RELiZORB outlet to the patient extension set or enteral feeding tube and manually prime through the device(s).
- If using a patient extension set, prime the feeding formula to the end of the patient extension set, and connect to the feeding port (button).
- Set the pump to the prescribed flow rate. Proceed with feeding.
Single RELiZORB setup for volumes up to 500 mL

Enteral pump flow rate: 10–120 ml/hr
Tandem RELiZORB setup for volumes 500-1000 mL
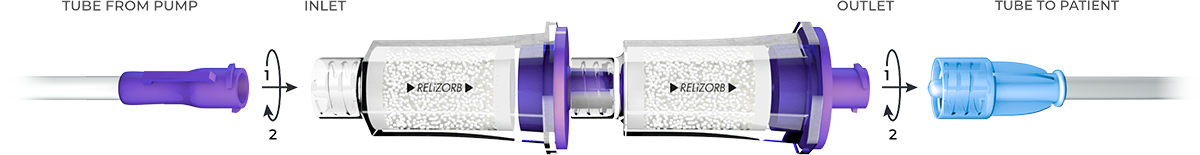
Enteral pump flow rate: 24–120 ml/hr
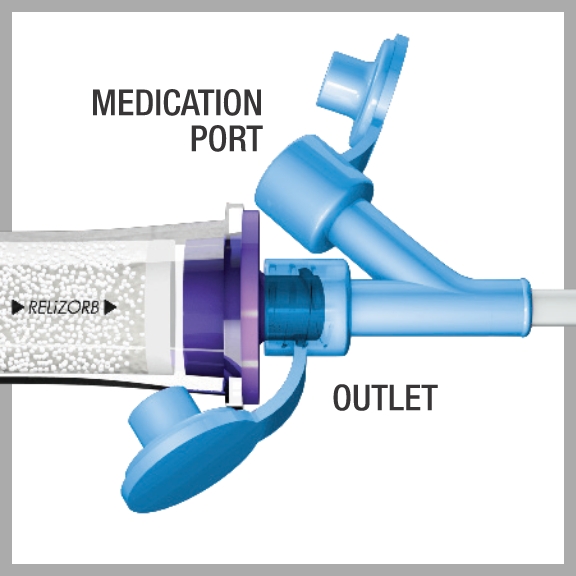
Medications should not be administered through RELiZORB
The passage of medications through RELiZORB may adversely affect the medications or the ability of RELiZORB to hydrolyze fats. To administer medications, use the medication port on an appropriate extension set after RELiZORB.
Helpful tips
- Please be sure to use RELiZORB exactly as prescribed by your doctor during each feeding. Do not make any changes to your feeding care plan without first speaking with your doctor or healthcare provider
- Medicines and saline flushes may only be added to the enteral feed line after RELiZORB. They may be added to the side port of a Y-Connector extension set located between RELiZORB and the patient (see image above)
- Examine the RELiZORB pouch. Do not use the RELiZORB if:
- The pouch seal is broken
- The current date is past the expiration date shown on the pouch
- Do not use the RELiZORB cartridge if:
- The RELiZORB is damaged
- The RELiZORB has been previously used
- When feeding is complete, disconnect the RELiZORB from the patient extension set or enteral feeding tube and enteral feeding pump tubing set. Throw away the RELiZORB after use in your trash
- RELiZORB has been tested for up to a 1-hour stop in feeding and shown not to change flow rates as measured by the flow of formula through the device (10-120 mL/hr for single RELiZORB, 24-120 mL/hr for tandem RELiZORB) or how well RELiZORB breaks down fat
For detailed setup instructions, please see the RELiZORB Patient Guide.
What is ENFit®? A safety standard in tube feeding connectors
ENFit is a connector designed for use in the tube feeding (enteral nutrition) system to help reduce the risk of enteral tube feeding misconnections and improve patient safety. The ENFit connector helps ensure that connectors do not connect to ports other than ENFit compatible tube feeding connectors. Download the RELiZORB ENFit Brochure to see how to connect RELiZORB with your tubing supplies.
Helpful ENFit tips
The Global Enteral Device Supplier Association (GEDSA) and The Oley Foundation recommend the following guidance for using ENFit supplies:
- When priming your pump, stop priming before fluid reaches the end of the tube.1 If any formula drips into the ENFit connector, wipe off any excess residue with gauze or tissue.2
- Keeping ENFit connectors clean and dry will prevent connections from becoming stuck together.2
- Be careful not to overtighten the ENFit connector.2
- Click here for more information on keeping your ENFit connectors clean.
After connecting your RELiZORB cartridge to the primed feeding tube, manually prime formula through the RELiZORB cartridge up to the outlet, then connect the outlet to the patient extension set.3
Reference
- Global Enteral Device Supplier Association (GEDSA). https://stayconnected.org/ENFit-cleaning-procedures-all-tubes. Accessed February 23, 2023.
- Oley Foundation. Lifeline Letter. September/October 2022:7-9.
- RELiZORB. Instructions for Use. Alcresta Therapeutics;2020.